Bravo Healthcare has put together and international consortium for development and production of vaccine against coronavirus 2019-nCoV. The consortium includes partners for preclinical development and validation, clinical trials, regulatory issues, production and distribution from the USA, India, Estonia , the Netherlands and Taiwan. All consortium partners are top-level experts in their area and are able and willing to work quickly and effectively for the common goal of making efficient coronavirus vaccine widely available. The consortium is ready to give the production licence to third parties according to request.
The Bravo consortium fully understands the challenge and the very tight time-line for developing and producing the vaccine against coronavirus. We also understand the necessity to keep the production costs of the vaccine as low as possible in order to make the vaccine widely available and stop the coronavirus epidemic. Therefore use a traditional and proven approach made more effective by our team. The method is fast, safe and cost-effective and easily scalable. Ideally we would be able develop the vaccine in 10 days, finish clinical trials in 1 month and the vaccine can be ready for end-users in max 2 months.
On Feb 14, 2020 the consortium presented an application for CEPI (the Coalition for Epidemic Preparedness Innovations).
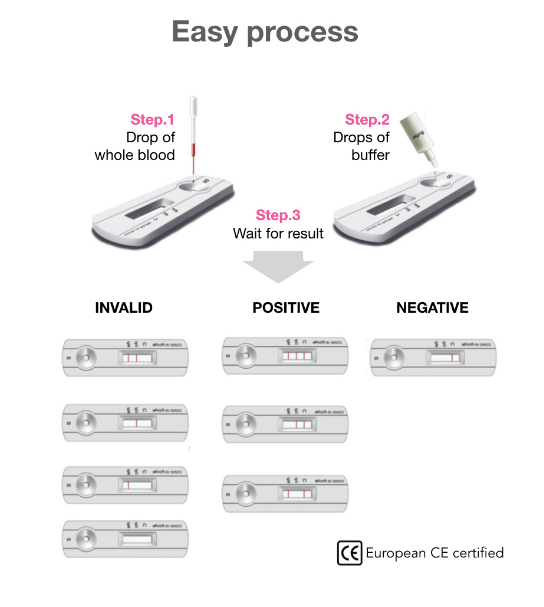
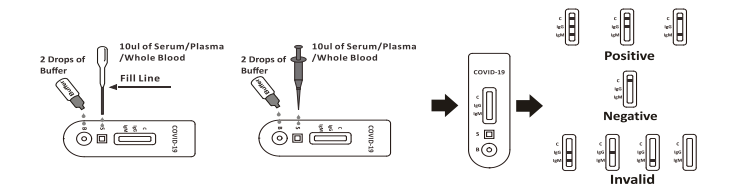
A rapid test for the qualitative detection of antibodies (IgG and IgM) against SARS-CoV-2 in whole blood, serum, or plasma.
According to an independent evaluation performed by the Pasteur Institute, France, the specificity of IgM is 99,2 %, the specificity of IgG 99,5 %, the sensitivity of IgM 91,8 % and the sensitivity of IgG 100 %.
The overall sensitivity reaches 100 % beyond 15 days.
Selectivity studies have shown an absence of cross-reactivity for a large panel of antibodies against a variety of viral or bacterial pathogens.
Full performance report will be available according to request by medical professionals from Riin Ehin (info@bravopharma.com).
The antibody-based tests can identify people who were not known
to be infected either because they never developed symptoms, or
they had symptoms that were never correctly diagnosed. That
means the test can identify silent infections, as well as people who
were once sick but have recovered.
Lei et al. have shown that antibody positive rates are very low in
the first five days after initial onset of symptoms, and then rapidly
increased as the disease progressed. Positive rates of viral RNA
however remained above 60% in the first 11 days after initial onset
of symptoms, and then rapidly decreased.
This demonstrates that serological assay is very important for
surveillance and control of the current COVID-19.
It is widely accepted that IgM provides the first line of defence during viral infections, followed by the generation of adaptive, high affinity IgG responses for long term immunity and immunological memory. Therefore testing of COVID-19 IgM and IgG antibodies is an effective method for the rapid diagnosis of COVID-19 infection. Furthermore, detection of COVID-19 IgM antibodies tends to indicate a recent exposure to COVID-19, whereas detection of COVID-19 IgG antibodies indicates a later stage of infection. Thus, this combined antibody test could also provide information on the stage of infection.